New mineral tanning system from Kemira
30 July 2012Kemira the water chemistry and treatment specialists headquartered in Helsinki, Finland, have developed Tanfor T, a universal tanning system free of chromium, aldehydes and aldehyde precursors. Tanfor T is a mineral tanning system, based on the natural elements aluminium and silicon. By Stefan van der Burgh
The tanning active compounds of Tanfor T are aluminium-silicon compounds. They are formulated together with organic acids and natural polycarboxylic acids that are perfectly safe for humans and the environment. Tanfor T does not contain volatile components and is only labelled as ‘irritant’. The raw materials are well known from everyday household products and water treatment products.
In addition, the ‘wet bright’ (tanned leather) intermediate that is produced will not yellow with age. Due to the mineral character of the new product, the leather offers a perfect dyeability and high dye affinity, allowing for very bright colours in all leather applications. In Figure 1, leather directly after tanning with Tanfor T is shown.
Tanfor T has been tested for shoes, garments, upholstery and automotive and meets all requirements for these applications. In Figure 2, a pair of safety shoes are shown, before being tested successfully for several months in their industrial production plant of propionates and acetates in Tiel, The Netherlands.
The tanning mechanism
In order to get a good tanning action, a tanning system needs the following characteristics: 1) penetration through the cross section without reaction, followed by 2) a reaction trigger that results in 3) an homogeneous fixation throughout the cross section. In order to achieve this Tanfor T was designed as a two-component system. As Tanfor T is mineral system, similar principles as with chromium tanning were followed.
Similar to salts of chromium(III) and other mineral compounds, the aluminium-silicon compounds that are the tanning active compound of Tanfor T are stable only in a certain pH range. At pH values above their stability range, the mineral salts will precipitate. At low pH, they are fully soluble, giving water clear solutions without signs of turbidity. Just below the maximum pH value of the stability range, a transition range is found where colloidal aggregates are formed. Colloidal aggregates are stable aggregates that will not precipitate, but are (much) larger than the single molecule of which they are formed. It is this colloidal aggregation state that is relevant for mineral tanning.
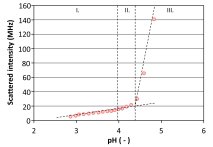
The relevant pH values and colloidal aggregation state of Tanfor T were mapped out using dynamic light scattering. Dynamic light scattering (also known as photon correlation spectroscopy or quasi-elastic light scattering) is a technique in physics that can be used to determine the hydrodynamic radius of small particles in suspension or polymers in solution. As the light source, monochromatic laser light was used and the scattering was measured at an angle of 90°. From scattering fluctuations, the Brownian motion and thus the hydrodynamic radius of the particles was calculated. The intensity of the scattering depends on particle concentration and especially particle size. Tanfor T was dissolved at pH 2.8 with the appropriate amount of salt to mimic tanning conditions. The pH was gradually increased by titrating small amounts of diluted NaOH into the system. After each addition, particle size and scattering intensity were measured. In Figure 3, the scattered intensity versus pH is shown. The region where colloidal aggregates were measured is indicated as region II and is found from pH 4.0 to 4.4. It was only in this region that colloidal size aggregates were measured. Above this region, the solutions became turbid and would precipitate with time. From these experiments, the conditions for an optimal tanning process with this product were derived. In order to formulate an effective tanning system around these aluminium silicon compounds, Tanfor T was developed into a 2-component system.
Tanfor T-A was formulated such that the pH will be around 3.5 when starting from a pickle pH3.2. At pH3.5, the hydrodynamic radius that was found was very small, below colloidal dimensions. These small particles do not react, allowing for a fast and unhindered penetration through the cross section.
Tanfor T-B was formulated such that a pH around 4.2 is reached as an overnight value. At this pH, the aluminium-silicon compounds bind to the collagen matrix. Also, the aluminium-silicon compounds will aggregate which creates bridges between the collagen fibres. After dosing Tanfor T-B, the pH in the hide will increase more slowly than in the float. This makes the locking reaction also slow, which allows for the aluminium-compounds of Tanfor T-B to also penetrate deeply into the collagen matrix, without excessive reaction on the surface or loss of material in the float.
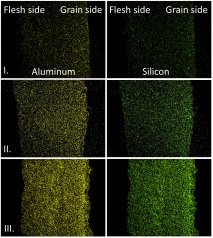
In Figure 4, the concentration profile of aluminium and silicon through the cross-section of a hide at the different stages of the tanning are shown as determined with scanning electron microscopy and energy-dispersive X-ray spectroscopy. With this technique, it is possible to carry out an elemental analysis of a sample. It is clearly seen in the pictures that both after the dosing of Tanfor T-A and Tanfor T-B, a very homogeneous concentration profile is obtained. No excessive surface reaction of tanning compound is seen in any of the stages of the tanning process.
Application
In practice, compared to a wet-blue process, only minimal changes are needed. The advised pickle is: Beaumé 5-7, float 40-80%, temperature 25°C, pH3.1. From there, it is dosed according to the scheme.
After applying this dosing scheme, no further basifying and/or buffering is needed as Tanfor T-B will have increased the pH to the proper basification pH. The final pH will be around 4.2.
This scheme will give a shrinkage temperature between 80 and 85°C directly after the tanning, as shown in Figure 5. Here, the shrinkage temperature was determined with differential scanning calorimetry. Additional to the shrinkage temperature, it is also seen that the slope of the curve is very straight, which indicates an homogeneous shrinkage temperature throughout the entire cross-section.
Leather characteristics
The ‘Wet Bright’ intermediate is firm and allows for a very precise shaving. Similar to chromium, it binds to the carboxylic groups of the collagen. The high cationic charge of the tanning compounds overcompensates the anionic charges of the collagen, making the leather cationic. This ensures a high uptake of retanning chemicals, fatliquors and dyes. Very bright colours are possible with Tanfor T with relatively low doses of dyestuff. The grain of leather produced is tight and flat.
Colour and lightfastness
The cationic character and even distribution of aluminium-silicon compounds in the ‘Wet Bright’ intermediate, together with the whiteness, provide an excellent substrate for colours. The red sparkles and is perfectly homogeneous through the cross section. Compared to conventional wet-white, the dye uptake is very high, and compared to chromium tanned leather, the colours are more sparkling due to the bright white base colour.
Post tanning
The amount of retanning and fatliquoring that is needed, is less than with conventional wet-white. In comparison to chromium tanning, only small fatliquoring and retanning adjustments are needed to get similar organoleptic properties.
Application
Due to the tight grain and absence of volatile components, Tanfor T makes an excellent automotive leather. The water resistance of leather made is better than leather based on chromium sulphate, providing an excellent basis for shoe upper. Due to the mineral character, with Tanfor T an excellent suede can also be produced in every colour possible. Bovine splits are easily upgraded into valuable suede without extensive mechanical operations.
Environment
Because of the environmental character of Tanfor T and the high exhaustion in tanning, the wastewater is very clean. The COD value with Tanfor T was determined compared with a wet-blue process and a conventional aldehyde based wet-white one.
Contact:
Stefan van der Burgh, Application Manager, ChemSolutions, Chemical & Pharma
Email: leather@kemira.com
Tel. +31 344 639 127
Mobile +31 6 21 177 743
Fax +31 344 611 475